Ledges annoy me. Like crazy. I finally put something together on how to remove them. Enjoy!
https://vimeo.com/279035347

Ledges annoy me. Like crazy. I finally put something together on how to remove them. Enjoy!
https://vimeo.com/279035347

Welcome to the first Endospot Case of Note. This case is of a C-Shaped lower molar and I’ve tried my best to highlight the interesting aspects of the case, explain what can be seen using our diagnostic tools, and also how to treat these cases. As usual, I would love to hear your thoughts and you can leave a comment below.
If you want to review the anatomical variations of C-shaped canals, I recommending reading the following references:
Fan B, Cheung GS, Fan M, Gutmann JL, Bian Z (2004). C-shaped canal system in mandibular second molars: Part 1 Anatomical features. Journal of Endodontics 30(899-903.
Fan B, Cheung GS, Gutmann JL, Fan W (2004). C-shaped canal system in mandibular second molars: Part II Radiographic features. Journal of Endodontics 30(904-8.
This video can be watched in HD on Youtube. Please enjoy!
httpv://www.youtube.com/watch?v=dF3JUsY_k8I

OK so a patient has come to see you complaining of toothache. It’s root canal time. You open up the lower right first molar and find a necrotic pulp. You’ve isolated well and done a nice endodontic preparation. Length is good and you’re happy with the sizes of your apical preps. You’ve been irrigating with plenty of sodium hypochlorite and used a final rinse of EDTA. Job done right?
Think again. In the vast majority of teeth, canals are not round, and as such there are going to be vast areas that are still harbouring debris and microbes. Just take a look at some of the microCT produced by Paque et al after preparation with rotary Ni Ti (Paque at al. 2010). It’s quite clear in these images that a good proportion of the original canal space remains untouched by our instruments.
So, hopefully, our irrigant is managing to get into these nooks and crannies? Well it will, but even after irrigation, there is still going to be significant debris from preparation (mushed up dentine, bacteria, necrotic pulp remnants) hiding away in fins, apical delta and especially in isthmuses.
I recommend you check out the work by Burleson and colleagues for some nice images on what can be left behind (Burleson et al. 2007). These guys conducted a randomised controlled trial (nice and high on the level of evidence) and compared preparation and irrigation alone, or with the addition of 1min of passive ultrasonic irrigation (PUI). They used the mesial roots of infected lower molars and then extracted the teeth to examine how much debris was left behind. Check out the table below to see the results on cleanliness of the canals at various levels from the apex. The isthmus especially is full of debris without PUI.

Cleanliness of Canals at various levels from the apex. Pay special attention to the number in the isthmus.
Interesting right? It’s even better when you see the images in their article and realise just how much is being left behind when you only rely on irrigation.
I know what you’re thinking though. It’s bacteria that we’re concerned about, not debris. Well, Burleson’s colleagues thought of this, and conducted a separate study, I assume on the same teeth, using microbiological sampling to see what they could find (Carver et al. 2010). They concluded that PUI resulted in a significant reduction in colony forming units and positive cultures. In fact PUI was, “7 times more likely to yield a negative culture”.
So, it seems that PUI is something worth doing, and it’s certainly part of my routine. If you haven’t heard of it, I’ll explain what it is. PUI is simply the placement of an ultrasonically activated file into the canal filled with irrigant. There is some debate over how it works exactly, but acoustic streaming seems to be the key. This basically means moving the solution around so that fresh sodium hypochlorite gets into areas that needles won’t push it. The important point here is that the ultasonic file has to be loose in the canal. Touch the canal wall and acoustic streaming stops.
Personally, I use the Irrisafe from VDW because it’s easy. Just screw it on to your US unit and place it in the canal. The irrisafe is “smooth” i.e. it doesn’t have the sharp cutting edges that files do, so it is safer in the canal (Hence the name – smart huh). Here are my three keys to PUI:
1. Use a low power setting as recommended by the manufacturer. You risk fracturing the ultrasonic file otherwise;
2. Keep the file loose in the canal and don’t push it too deep;
3. Replenish your sodium hypochlorite as you go.
I generally have my DA using a syringe to supply sodium hypochlorite and suction while I run the ultrasonic in the canals. If you don’t have a stand alone ultrasonic unit where you can easily control the power, then I wouldn’t risk using an ultrasonic file. You can achieve the same result by just holding a normal scalar tip against a size 10 stainless steel file. This is obviously much cheaper as well. Just be careful not to put the file too deep into the canal or you might actually damage the canal walls and ruin your nice preparation.
There are other methods for activating irrigant such as the Endoactivator and the Endovac. These work slightly differently. The Endoactivator is a sonic machine and has disposible polymer inserts. It seems very safe to me, but there isn’t much in the literature indicating that it is better at killing bugs than other methods (Huffaker at al. 2010), and it is not as effective as PUI at removing calcium hydroxide from canals (Wiseman et al. 2010).
The Endovac shows promise. It relies on a vacuum to remove irrigant that is being supplied by a needle deep in the canal. That means there is no positive pressure. One clinical trial showed that post-op pain was reduced after using the Endovac compared to needle irrigation (Gondim et al 2010). It is a bit of a contraption though and I have enough machines clogging up my surgery. I am waiting to see some studies comparing PUI directly to Endovac and Endoactivator before I give up my proven method of cleaning the canals.
Next time you finish a prep, grab your ultrasonic and give the canal a bit of a shake up. It’s impressive to see how much more debris you can get out when you irrigate again.
References:
BURLESON A, NUSSTEIN J, READER A, BECK M. 2007. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. J Endod. 33: 782-787
GONDIM E, SETZER F, BERTELLI C, KIM S. 2010. Postoperative Pain after the Application of Two Different Irrigation Devices in a Prospective Randomized Clinical Trial. J Endod. 36:1295–1301
HUFFAKER S, SAFAVI K, SPANGBERG L, KAUFMAN B. 2010. Influence of a Passive Sonic Irrigation System on the Elimination of Bacteria from Root Canal Systems: A Clinical Study. J Endod. 36:1315–1318
PAQUE F, BALMER M, ATTIN T, PETERS OA. 2010. Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: a micro-computed tomography study. J Endod. 36(4): 703-7
WISEMAN A, COX C, PARANJPE A, FLAKE N, COHENCA N, JOHNSON J. 2011. Efficacy of Sonic and Ultrasonic Activation for Removal of Calcium Hydroxide from Mesial Canals. J Endod. 37:235– 238

In this post, we discuss a simple and practical method of classifying pulpal pathology that can be used in practice on a daily basis. For the new and updated Endospot Podcast, go to the Podcast Page.
Listen to the episode here (12 minutes):The Endospot Episode 2 | Simple Guide to Pulpal and Periapical Diagnosis Part 1
BARBAKOW F, CLEATON-JONES P, FRIEDMAN D. Endodontic treatment of teeth with periapical radiolucent areas in a general practice. Oral Surg Oral Med Oral Pathol 1981. 51, 552–559.
BENDER IB. 2000 Pulpal Pain Diagnosis. A Review. J Endod 2000. 26, 175-179
BENDER IB. 2000. Reversible and irreversible painful pulpitides: diagnosis and treatment. Aust Endod J 2000. 26, 10–14.
MILES TS. Dental pain: self-observations by a neurophysiologist. j Endod 1993. 19, 613-5.
SELTZER S, BENDER IB, ZIONTZ M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol 1963. 16, 846-71 and 1963. 16, 969-77.
SIGURDSSON A. 2003. Pulpal Diagnosis. Endodontic Topics. 5, 12-25.
Transcript:
Welcome to the Endospot Episode 2.
My Name’s Pat Caldwell and today we’re coming to you from Shanghai, China. On our website we have the complete list of references discussed in this episode, so go to www.endospot.com and remember you can sign up online to receive the Endospot directly to your inbox.
In this episode, we’ll be discussing the classification of pulpal pathology. This will be the first in a series of three episodes looking at the issue of diagnosing pulpal and periapical pathology and in this episode we’ll go through a useful classification system which will help you deliver appropriate treatment to your patients. I’ve broken it up into three episodes because diagnosing and therefore treating appropriately is just so important. I think that often not enough time and thought goes into the initial diagnosis and that is when errors are made and our patients suffer. As endodontists we often see cases where an incorrect diagnosis has been made and treatment either withheld or provided when it wasn’t required and as professionals we owe our patient more than that.
When we look at the pulp and periapical classification systems, there are many out there, but in my opinion the simplest and most useful is the one proposed by Asgir Sigurdsson (Sigurdsson 2003). He published an excellent journal review article on this particular topic in everyone’s favourite journal, Endodontic Topics and that’s the classification system I’ll be discussing today. This classification is very much designed to give a clear indication of the treatment requirement according to the diagnosis, and when it comes down to it, that’s what we’re really looking to achieve out of our diagnosis.
Now when I’m faced with a new patient, whether they are presenting in pain or not, I will still conduct a complete history and examination and make both a pulpal and periapical diagnosis. I split the diagnosis into pulpal and periapical because it helps me have clear in my mind exactly what I’m dealing with, how we should proceed and what follow up’s required. It will also give us clues as to what problems we might run into, such as if the tooth will be difficult to anaesthetise, or if there’s likely to be a high level of post-operative pain. To be fair it will most often be the pulpal diagnosis that is driving your treatment decisions and that’s another reason to spend an episode on going through the various diagnoses.
Now if you think about it, the only way we can definitively diagnose the state of a pulp is to extract the tooth and slice it up and look at it through a microscope. So, our clinical diagnosis is always going to include an element of a guess and I can assure you that it will not always be straightforward. But I dIgress, we’ll get onto how to diagnose in a future episode so now let’s get back to the topic at hand. The classification system we are going to be using includes 4 different diagnoses for the dental pulp, and one of them is the healthy pulp. That’s the easiest one so we will start with that one.
A diagnosis of healthy pulp assumes that the pulp is vital and is not inflamed in any way. This tooth will respond normally to pulp testing, that is hot, cold and electric. It will be asymptomatic and shouldn’t be tender to percussion or palpation unless there is an occlusion issue causing this. We use this classification sometimes when we need to do RCT on a tooth for prosthodontic reasons. So an example might be where there is not a lot of coronal tooth structure remaining in a vital tooth and the restorative dentist feels that an intracoronal restoration is required in order to provide retention for a core.
We’ll now move on to the more complex diagnoses, the ones indicating some sort of inflammatory response. The first of these is Reversible pulpitis. If we looked at this pulp under a microscope, we’d see a vital pulp with areas of localised inflammation. Most commonly this will be associated with a response to caries or possibly microbial leakage of a restoration. It could also be due to exposed dentine or bacterial ingress along a crack. All these things will lead to inflammation in the pulp. Now by definition, this inflamed pulp should heal when we remove the cause of the inflammation, so it’s important not to misdiagnose this pulp with an irreversible pulpitis and initiate RCT when it’s not needed.
A pulp with reversible pulpitis can present with quite a broad range of symptoms. Typically, the patient will describe pain with hot or cold, or with biting in the case of a crack. The pain might be mild, but can sometimes be severe, but probably the key to this diagnosis is that removal of the stimulus will lead to rapid relief of the pain. For example, drink something hot, tooth hurts, swallow the drink, tooth is fine. In general, there should also be no report of spontaneous pain, and no tenderness to percussion or palpation. The tooth is likely to respond to a pulp test, but the inflammation in the pulp might mean that an exaggerated response is gained. This in itself isn’t an indication of irreversible pulpitis, if the other indicators are that of reversible inflammation.
When you diagnoses reversible pulpitis and remove the stimulus by removing caries, or covering exposed dentine, it’s important that you review the patient and re-do all the examination procedures. Because if you think about, if you’ve diagnosed reversible pulpitis, say in case where there is a carious lesion and you treat it by removing the caries and placing a restoration, but the symptoms remain then by definition the pulp wasn’t reversibly inflamed.
Choosing between a diagnosis of reversible pulpitis, and it’s for more unpleasant alternative irreversible pulpitis is a critical decision, because the treatment options are very different. Where a pulp is irreversibly inflamed, this means that the inflammation is so severe that the pulp will not be able to heal. It will eventually necrose and become infected, leading to apical periodontitis. The treatment for these teeth is to undertake root canal therapy in order to remove the diseased pulp tissue and prevent infection.
As with reversible pulpitis we see a wide range of presentations. Commonly there is an exaggerated response to hot and cold, but the key here is that this response lingers for some time. It’s hard to say exactly how long it has to linger to be considered irreversible, so here I’ll have to take some licence and say that a pain that lingers for a number of minutes after stimulus is not a healthy pulp. There is a good biological explanation for this lingering pain but I’ll go through that with you in the diagnosis episode. Sigurdsson tells us to be careful when interpreting this symptom as if you put an intense stimulus even on a healthy pulp the resulting pain will linger somewhat.
Another indicator that we’re dealing with an irreversibly inflamed pulp is where the pain has been severe, and the longer it has been present, the more likely it is to be irreversible in nature (Bender 2000). When pulp testing this tooth, there will often be an exaggerated response and the dull lingering pain experienced before will be induced by the procedure. Tenderness to percussion is often also present. The level of inflammation in these teeth will often lead to neurogenic pain and nerve sprouting which results in inflammation in the periapical tissues. This can occur well before the pulp starts to die and therefore even in a vital, inflamed pulp, tenderness to percussion is often present.
Probably the biggest indicator of an irreversibly inflamed pulp is spontaneous pain. So the patient will report moderate to severe pain that suddenly occurs, and often remains as a dull lingering pain for minutes or hours. They might even report being woken by the pain. I often find that patients with irreversible pulpitis have resorted to over the counter painkillers such as ibuprofen and report that these drugs will help relieve the pain until they wear off of course.
There is an excellent article published by Timothy Miles, who is a neurophysiologist who had previously trained as a dentist (Miles 1993). In the article he explains in detail his not only the physiological response he personally experienced due to a dying pulp, but also the emotional response. It has a grand total of 6 references which it probably could have done without and yet was published in the Journal of Endodontics. It’s probably essential reading for any dentist who has never experienced toothache, and I’ll put the reference in the show notes. If you ever sit the examinations for the Royal Australasian College of Dental Surgeons, then I believe he still lectures on the primary orientation course, and you can have the pleasure of hearing the story in person.
Our final diagnosis is pulpal necrosis. This covers both partial and complete necrosis of the pulp. Consider the progression from reversible pulpitis to irreversible pulpitis and on to pulpal necrosis. At some point the inflammation builds to a level where the vital tissue dies. This necrosis over time then spreads throughout the whole pulp space. There isn’t a lot of information on exactly how quickly the process occurs and I imagine that there is a great variation in the length of that process, but when it comes down to it, some form of Root canal therapy is required.
Most of the time when a pulp is necrotic, it will also be infected. There are a few situations where a pulp can necrose and remain uninfected, mostly after physical trauma to a tooth, but on the whole, a dead pulp lacks a blood supply and therefore lacks the ability to protect itself against microorganisms. These bugs will get into the pulp space through cracks or infected dentine, or possible exposed dentinal tubules and rapidly take over the necrotic space.
When dealing with a patient presenting in pain, there is a strong correlation between the following factors and pulpal necrosis. First is a history of moderate to severe pain. Second is tenderness to percussion, third is a history of spontaneous pain and fourth is a negative pulp test. Seltzer and Bender conducted some of the most useful research we have on this topic way back in 1963 (Seltzer et al. 1963). Basically, they examined patients who presented with pain and then the tooth was extracted and examined histologically. There is a nice summary of their findings in a paper published by IB Bender in 2000 in the JOE which you should read if you are a postgraduate student (Bender 2000).
It’s important to note here though that very often the progression from vital to necrotic pulp is painless, or the level of pain is minimal and no help is sought for the problem. Often patients are completely unaware that they have a chronic infection in their jaw. Two studies reported this happening in 26-60% of cases. (BARBAKOW ET AL. 1981, BENDER 200) In my experience these teeth may be completely asymptomatic. They can be slightly tender to percussion but often don’t even present with this. Usually, they are identified due to a lucency on a PA or OPG Xray. The key for these though is that they will not respond to a pulp test and can be entered without local anaesthetic.
OK, I think that’s enough for this episode. Next episode we’ll be discussing the various periapical diagnoses that we can make. In the meantime, I recommend you have a look at the references, and next time and every time you work on a tooth, have a think about what diagnosis you have for the pulp. If you’re placing large restoration or crown think about whether you’re completely confident of your diagnosis before doing the work.
Also, if you’ve got a comment, or you disagree with something that was said here, please keep it nice, but I’d love you to go to the blog and leave a comment.

In this, post, we discuss practical techniques for preventing failure of LA for endodontics, especially when dealing with irreversible pulpitis. Listren to the audio file below:
Preventing Local Anesthetic Failure in Endodontics
For the new and updated podcast, go to the podcast page.
Meta Analyisis on Artciaine V lidocaine:
KATYAL V. 2010. The efficacy and safety of articaine versus lignocaine in dental treatments: A meta-analysis. J dent: 38: 307-317
Intraossesous Injections:
READER A, NUSSTEIN J. 2002. Local anesthesia for endodontic pain. Endodontic Topics: 3: 14–30
Use of Articaine for buccal infiltration:
MATTHEWS R, DRUM M, READER A, NUSSTEIN J, BECK M. 2009. Articaine for Supplemental Buccal Mandibular Infiltration Anesthesia in Patients with Irreversible Pulpitis When the Inferior Alveolar Nerve Block Fails. J Endod: 35(3): 343-346
AGGARWAL V, JAIN A, KABI D. 2009. Anesthetic Efficacy of Supplemental Buccal and Lingual Infiltrations of Articaine and Lidocaine after an Inferior Alveolar Nerve Block in Patients with Irreversible Pulpitis. J Endod: 35(7). 925-929
PSA Block Link –
Information on the PSA Nerve: http://en.wikipedia.org/wiki/Posterior_superior_alveolar_nerve
Video: You Tube Video on the PSA Nerve Block
Show Transcript:
Welcome to the Endospot Episode 1.
In today’s podcast I’ll be discussing my techniques for managing local anaesthetic failure and specifically how to manage teeth diagnosed with irreversible pulpitis.
On the site we have references and show notes for this episode, so head to www.endospot.com and remember you can sign up and receive the Endospot blog posts directly into your inbox.
So let’s get on with the podcast.
On the topic of failed LA, it’s important to recognise situations where complete anaesthesia is likely to be difficult to obtain. In general terms, the mandibular molar teeth are the most difficult to anaesthetise, and this is where you’re likely suffer the most failures.
The issue with mandibular molar teeth is compounded where we have an irreversible pulpitis. The inflamed pulp is always going to more difficult to anaesthetise. You’ll often have a slightly distressed patient in this situation, so it’s important to get it as right the first time. Once you’ve begun treatment on an anxious patient and they’ve suffered a level of pain due to failed LA, it can be quite difficult to win back their faith in you.
Now it’s certainly my experience that where the pulpitis has been long standing and is becoming more and more acute, this will be the most difficult tooth to get numb. A good example might be a case of cracked tooth syndrome where the tooth has been manageably painful to bite for months, but is now becoming more and more sensitive to thermal stimuli and spontaneous pain has begun to occur. I suggest you hit these ones with every trick in the book.
Abscessed teeth don’t normally represent a huge problem for anaesthesia in terms of being able to instrument the canals, but sometimes the surrounding tissues will be difficult to anesthetise and the patient will still respond to things like pressure. One caveat here though. Just because you’ve made a diagnosis of abscess, don’t assume that there is no vital tissue in the canal. Some vital tissue can remain in the canal even in the presence of pus and swelling, and attempting to debride this canal can result in high levels of pain. So, I always assume the worst when dealing with patients in pain and provide complete anaesthesia
In my previous last post, I covered the reasons for LA failure in pulpitis cases, so if you want more details and references, go to the blog post at endospot.com and review those there. The main points that are worth considering though, are that a numb lip after IANB does not necessarily equal pulpal anaesthesia. This is the case even when dealing with an uninflamed pulp, but is especially so where you are dealing with an inflamed pulp. Research generally tells us that if we are dealing with an irreversible pulpitis and we give an IAN block which is apparently successful as the patient’s lip is numb, then , we’ll still only be successful in completely anaesthetising the tooth nerve about 55% of the time. Now, that’s obviously unacceptable, so we’re going to need to use supplementary LA for these cases.
The second point to note is that LA takes time to work. Again, research tells us that in 19-27% of cases, complete anaesthesia doesn’t occur until 15 minutes after the injection. So, next time your LA fails, it may simply be that the LA hasn’t had time to work. Supplemental techniques such as the PDL and intraosseous injection tend to work much faster than IADB, and should have you removing that inflamed pulp much faster.
As to which type of LA you should use, this will to a certain extent come down to personal preference, and obviously the medical profile of the patient. In general terms though, I would recommend using articaine. There’s been plenty of research in recent years devoted to articaine with some conflicting results. But, a recent meta-analysis concluded that articaine was more effective than lidocaine for anaesthetising mandibular molars. The article is by an Australian and so refers to lignocaine, but I assure you it’s the same drug.
So now I’ll go through my standard technique for dealing with hot pulps. Wherever not contraindicated, I’ll prescribe ibuprofen as soon as possible prior to treatment. There is some evidence that this alone may assist in achieving complete anaesthesia in inflamed pulps, but even if it doesn’t let’s remember that these patients are presenting with pain, and therefore are at risk for post-operative pain. In this case, Ibuprofen (unless contraindicated) would be part of my pain management regime anyway.
When faced with a hot mandibular molar, I’ll start with a regular IAN Block and follow this with a buccal and lingual infiltration. The buccal infiltration ensures that a rubber dam clamp can be placed comfortably, but more importantly, I think that combining the block of the nerve with the local infiltration simply means that we have two sites along the nerve trunk that are blocked and this will lead to improved anaesthesia. This makes a lot of sense to me and is backed up by a number of studies which show that in cases of irreversible pulpitis, the infiltration significantly improves success rates. These studies have also shown the improved effect of articaine over lidocaine for this purpose.
After the buccal and lingual infiltrations, we take a minute’s break to ensure they are active and then move onto the PDL injection. There are a number of specialised equipment kits for PDL injections available, but they can be done successfully with a standard syringe. The first step is to bend the needle with some haemostats so that you are able to place pressure on the needle as it is forced into the PDL. The needle is inserted into the gingival crevice and with the bevel facing away from the root surface. Press hard and inject for 10s. There should be back pressure, if there isn’t, then the solutions wont’ be forced through the cribriform plate into the cancellous space and won’t be effective. I’ll perform the PDL injection at the four corners of the tooth.
The PDL injection is not really a PDL injection. I mentioned that the solution is forced into the cancellous space and as such It’s really an intraosseous injection. Therefore, there may be cardiovascular effects and this should be taken into account when selecting the LA. If an adrenaline containing LA is contraindicated, then you can use a non-adrenaline containing LA for this purpose.
The rate of onset of a PDL injection is fast, but the duration is short compared to IAN block, so at this point I’ll get a rubber dam on to the tooth and start access almost immediately. Once the pulp space is penetrated, and if vital tissue is found, then I’ll perform an intrapulpal injection. This injection relies on backpressure, so can only be used if only a small perforation is made into the pulp chamber.
For maxillary molars, I’ll start with a buccal and palatal infiltration, injecting most of the cartridge bucally. The palatal injection allows the rubber dam clamp to be placed comfortably, but might also anaesthetise the palatal root where a buccal infiltration could fail. After this, I perform a posterior superior alveolar block. I’ve placed a link in the show notes so you can have a look at this technique if you’re not familiar with it. I think that in the same way that the buccal infiltration in the mandible works with the IAN block to improve anaesthesia by blocking two points along the nerve trunk, combining a SPAN block with infiltrations should do the same. I then move onto PDL injections and perform an intrapulpal once the pulp chamber is entered.
The vast majority of cases, these procedures will allow you to enter the pulp space and remove most of the inflamed pulp tissue. If you are unable to even enter the pulp space, then your only real option is to perform an intraosseous injection. This does require specialised equipment. A good review of this technique can be found in the endodontic topics article referred to in the show notes. If you are comfortable with intraosseous injections, then these can be substituted for the PDL injections in the standard routine. My practical experience though is that if you follow the techniques described you really should have a very high level of success.
One point to note though is that infiltrations and PDL injections won’t make up for a failed IANB, so if there is any doubt that the block has been successful, then it’s worth repeating the block. If you want to be certain, it might make sense for you to perform the block and then wait until there are significant signs of lip and tongue anaesthesia before going onto the infiltrations and PDL injections. Obviously these injections can cause lip numbness also and so can mask a failed IAN block.
After entering the pulp chamber, you may find that you can remove some or most of the pulp tissue but attempting to pass a file to length in the canal elicits pain. In this case, I don’t see any reason to persist as the process of removing the bulk of the pulp tissue will result in resolution of the patient’s symptoms. I’ll dress the tooth with a corticosteroid containing dressing such as ledermix or odontopaste and send the patient home with assurances that the symptoms will resolve and the tooth will be easier to treat at the subsequent visit as the inflammation in the pulp will have resolved greatly. If you persist when complete anaesthesia isn’t achieved, you’re likely to put your patient and yourself through a lot of unnecessary pain, and the patient will be sure to be more anxious and will probably be one of those people that for the rest of their life tells their friends how agonising root canal treatment is.
So that is my routine for dealing with hot pulps. I’m aware there will be many different techniques out there and I’d love to hear some feedback. For those listeners who are just beginning their career, I’d recommend getting into the habit of using block and infiltration anaesthesia for all endodontic cases, and adding the PDL and intrapulpal injections when required.
Podcast: Play in new window | Download

Every dentist has had the unfortunate experience of being unable to achieve anesthesia, especially when dealing with an irreversibly inflamed dental pulp. You find yourself filled with self-doubt and feeling helpless, especially where the patient has presented in pain and you feel you need to remove that inflamed pulp!
But, LA failure is a bit more complex than I think most of us realise. Most of the information that follows is taken from an excellent review of the topic by Ken Hargreaves & Karl Keiser, so grab that if you want in depth analysis (Hargreaves et al. 2002). I’m going to cover what I think is important for most of us.
There are a number of reasons for LA Failure, but the first thing I think we should consider is this:
1. Complete pulpal anesthesia is not achieved 100% of the time in normal pulps;
2. Complete pulpal anesthesia has a slower onset than most of us would expect .
The simple fact is that a well administered inferior alveolar nerve block does not provide complete pulpal anesthesia 100% of the time. Bou Dagher conducted IAN block on 30 subjects and achieved 100 numbness of the lip, but only 50-75% of these patients demonstrated complete molar anesthesia when measured by electric pulp tester (Bou Dagher 1997). For most dentists, the majority of LA is given for restorative procedures, and often, anesthesia may be sufficient to prepare a cavity for restoration without problem. I think this probably lulls us into a false sense of security as to how successful our techniques are.
The next point to make is that the onset of complete pulpal Anesthesia takes time, most commonly up to 15 minutes. It’s fair to say that we would often not wait that long before attempting a procedure. In studies, onset was longer than 15 minutes in 19-27% of cases and longer than 30 minutes in 8% of cases. Complete lip numbness occurred much faster, within 5-7 minutes.
On this topic, It has been shown that injecting a second cartridge of LA injected after an apparently successful IAN block does not improve the onset time (Vreeland et al. 1989). In those situations where we have an apparently failed IAN block (despite profound lip numbness), and we inject a second cartridge and we then achieve anesthesia, it’s probably just that we’ve allowed more time for the initial injection to work.
The key points here are:
1. Lip numbness does not confirm pulpal anesthesia;
2. Onset takes time
3. IAN blocks are unreliable when it comes to achieving pulpal anesthesia even in uninflamed pulps
What happens when we have an inflamed pulp? Things are even more dire. Two studies that evaluated pulpal anesthesia after IAN where the pulp was inflamed reported an average of only 55%, despite 100% numbness of the lip (Cohen et al. 1993, Nusstein et al. 1998).
Theories on failure of LA
It is my recollection from reading textbooks at dental school that IAN blocks failed because of the increased pH in the region of an inflamed pulp which prevented dissociation from the acid form of the LA to the base form. The base form was then unable to diffuse across the cell membrane in order to block the sodium channel. This never sat well with me because I couldn’t understand why inflammation at the site of a lower canine tooth would affect the dissociation of an LA molecule deposited near the mandibular foramen. The truth is that while this theory remains a possibility, it is unlikely that this is a real explanation for the failure of LA. Firstly, the change in pH in inflamed tissues is not large, and inflamed tissues possess a greater buffering ability than normal tissues. Secondly, any change in tissue pH is also likely to be very localised (Punnia-Moorthy, 1988). So, given our clinical results of reduced anesthesia of mandibular teeth after IAN blocks, we can consider this explanation to have some, but probably limited clinical relevance.
The second reason I recall from dental school for failure of IAN blocks was accessory innervation from the myelohyoid nerve. The lingual, buccal and transverse cervical nerve have also been implicated, however there is limited evidence for these mechanisms. Potentially, however, they could contribute to LA failure.
A third possible method mentioned in the literature is resistance or tachyphylaxis. According to Kenneth Hargreaves (who by the way is an extraordinary speaker and you should make an effort to see him speak) there is little evidence for this, and I think we should be happy to take his word for it.
The next possible mechanism of failure of LA is through the increased blood flow that occurs in inflamed tissues. This could potentially result in increased removal of LA from a site. Again, this is likely to be a localised issue and should not affect regional block anesthesia. Theoretically, increasing the concentration of vasoconstrictor in LA should counteract this mode of failure, but to date there have been few studies (I couldn’t find any) comparing different concentrations of adrenaline in pulpitis cases. In normal pulps, the results have been a little contradictory with some studies showing equivalent results for different concentrations of adrenaline (Epinephrine). One study however has shown a dose dependant effect on onset and duration of infiltration anesthesia with 2% lidocaine and 1:200000, 1:100000 and 1:50000 adrenaline.
OK, now we get into the important stuff – the real reasons why we have trouble convincing inflamed pulps to be quite while we operate on them. In the presence of inflammation, a number of changes occur to the actual nociceptors, or pain receptors. The receptors become both activated and sensitized. For example, when bradykinin is released, this will cause the neuron to fire, causing pain.
Sensitization of nociceptors occurs due to other inflammatory mediators, such as prostaglandin E2 (PGE2). This results in the threshold for the nerve firing reducing. The result is that the nociceptors will activate with a much milder stimulus. It has been shown that both activation and sensitization result in a level of resistance to anesthetics (Rood et al. 1981).
Another thing that occurs in response to inflammation that might surprise you is nerve sprouting. Inflammatory mediators actually cause nerves to grow into the inflamed are and this has been shown to happen in human dental pulp (Byers et al. 1999). This simply means there are far more nociceptors to block and results in an increased receptive field. Check out work by Byers for some great microscope images showing the vast increase in nociceptors in inflamed rat pulps.
Inflammation also causes an increase in the production of proteins by nociceptors, such as substance P and calcitonin gene related peptide. These proteins play a role in the regulation of inflammation in the pulp, and may have some role to play in LA failure.
Another interesting concept relates to a specific type of sodium channel on neurons which is resistant to tetrodotoxin (TTX), funnily enough called the TTX-resistant sodium channel. These channels are less sensitive to lidocaine, about one quarter as sensitive as normal sodium channels and are present on human pulp nociceptors under normal conditions. Expose a nerve to PGE2, and their activity doubles (gold et al. 1996). Therefore, this represents a mechanism whereby LA could fail. My thoughts are that the prostaglandin might only operate on these channels locally, and this theory might suffer from the same argument as the pH change theory, that is that it might only occur locally, so shouldn’t necessarily effect block anesthesia.
Central Nervous System (CNS) Sensitization may contribute to LA failure. When inflammation and pain occur for an extended period of time, this results in an increased excitability of the CNS. In basic terms this means that a lesser stimulus will result in a higher level of pain being experienced. Central sensitization is a blog post all by itself and as an endodontists, we are particularly careful of pain control in any patient who presents with a history of chronic pain. Hargreaves feels that the excited CNS may be responsible for otherwise innocuous stimulus presenting as anesthetic failure. For example, we may treat a patient who feels some minor discomfort but tolerates it for the procedure. In a patient who has been subject to central sensitisation, the discomfort may present as pain.
So there you go, and I hope this makes you feel a bit better the next time you can’t anaesthetize a “hot” pulp. It’s probably a bit more complicated than you previously thought, and it’s probably a bit less your fault than you previously felt. I’ll be discussing my methods for dealing with LA failure and inflamed pulps in a separate blog post.
BYERS MR, NARHI MVO. 1999. Dental injury models: Experimental tools for understanding neuro-inflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol
Medical . 104–139.
BOU DAGHER F, YARED G, MACHTOU P. 1997. An evaluation of 2% lidocaine with different concentrations of epinephrine for inferior alveolar nerve block. J Endod. 23: 178–180.
COHEN HP, CHA BY, SPANGBERG LS. 1993. Endodontic anesthesia in mandibular molars: a clinical study. J Endod. 19: 370–373.
GOLD M, REICHLING D, SHUSTER M, LEVINE JD. 1996. Hyperalgesic agents increase a tetrodotoxin-resistant Na¹ current in nociceptors. Proc Nat Acad Sci: 93: 1108.
KENNETH M. HARGREAVES & KARL KEISER. 2002. Local anesthetic failure in endodontics:
Mechanisms and Management. Endodontic Topics 2002, 1, 26–39
KNOLL-KOHLER E, FORTSCH G. 1992. Pulpal anesthesia dependent on epinephrine dose in 2% lidocaine. Oral Surg Oral Med Oral Pathol. 73: 537–540.
NUSSTEIN J, READER A, NIST R, BECK M, MEYERS WJ. 1998. Anesthetic efficacy of the supplemental intraosseous injection of 2% lidocaine with 1: 100,000 epinephrine in irreversible pulpitis. J Endod. 24: 487–491.
PUNNIA-MOORTHY A. 1988. Buffering capacity of normal and inflamed tissues following the injection of local anesthetic solutions. Br J Anaesth. 6: 154–159.
ROOD JP, PATEROMICHELAKIS S. 1981. Inflammation and peripheral nerve sensitisation. Br J Oral Surg 19: 67–72.
VREELAND D, READER A, BECK M, MEYERS W, WEAVER J .1989. An evaluation of volumes and concentrations of lidocaine in human inferior alveolar nerve block. J Endod 1989: 15: 6–
12.

Below is a video of a demonstration I gave at the Jiao Tong University Dental School in Shanghai. I was demonstrating a method for removal of GP from treated canals. The method that I describe is only one of many methods, but I think it is a good way to begin re-treating. Please watch the video and share your thoughts:
httpv://www.youtube.com/watch?v=D5SeDNOusV4
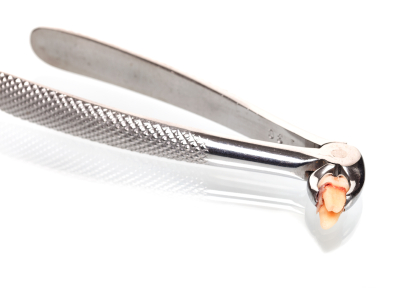
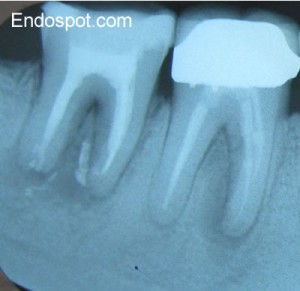
Despite Endodontic Re-Treatment The Second Molar Was Associated with a Sinus Tract. Resorption of the Distal Root Apex Can be Seen.
Intentional Reimplantation involves carefully extracting a tooth, performing root end resection and retro fill, and reimplanting the tooth. It is a simple and reliable procedure which can be performed by any dentist and can be used to retain teeth that might otherwise be deemed hopeless. See the Endospot Study Guide to Intentional Reimplantation at the end of this post for references and a full list of indications/contraindications etc.
In situations where endodontic procedures have failed or cannot be undertaken, endodontic surgery is a commonly used treatment modality. There are, however, situations where traditional endodontic surgery is difficult, or inappropriate. In particular, this occurs where anatomy such as a thick bony plate limits access (especially in the lower 2nd/3rd pre-molar), or vital structures such as neurovascular bundles are at risk (especially the mental n. in the region of the lower 1st/2nd molar). This also occurs due to other factors such as accessibility, lack of patient cooperation or a preference not to undertake surgical procedures.
In these cases, intentional reimplantation (IR) may provide an option to treat persistent apical disease. In certain cases, IR is the preferred option over surgery. IR is not a last hope procedure. When done according to protocol it is very predictable. The procedure itself is something that many dentists may find confronting, but in reality it is not difficult to do, and most dentists can have it in their repertoire.
I’ve mentioned that IR is not a last hope procedure, but in any event, when you have run out of options, and a tooth is to be extracted, then it may be worth considering treating and replanting the tooth.
The two main contraindications to IR are flared or curved roots and periodontal involvement. The first is an obvious contraindication, because the tooth needs to be able to be extracted in a controlled manner. One key factor for success is to limit the damage to the cementum layer of the toot. If the cementum layer or PDL is damaged, then there is a greater potential for resorption to occur. The second is obvious as well, because an unhealthy periodontium will complicate the healing process. Once again, see the study for a full list.
When I discuss this procedure with dentists, they are often concerned about the potential for resorptive processes and ankylosis. Most of this concern comes from experience with teeth that have been avulsed. IR is a completely different situation to a traumatic avulsion. You could reasonably expect far more trauma to the cementum and bone during a traumatic avulsion, but probably more importantly is that the tooth is often left , contaminated, dry and out of the socket for an extended period. This leads to necrosis of the PDL, and ankylosis ensues. With IR, the tooth is very carefully extracted and maintained out of the mouth in a moist environment for a very limited time. These conditions are ideal to allow healing.
A classic case is the lower second molar which has been treated endodontically and shows signs of persistent disease. In the case below, the patient was referred for management of persistent disease in the first molar. The lower right second molar has been re-treated by and endodontist 3 months prior to the patient being referred to me. Unfortunately, there was a sinus tract present, associated with the distal root of the 2nd molar (determined by taking a radiograph with a #30 GP point in the tract).
I spoke to the treating endodontist who told me that the distal root had an open apex, and he had filled the distal canal with MTA. In this case, access and a large amount of bone overlying the root of the 2nd molar make surgery untenable. Even many experienced endodontists will tell you they have never performed traditional apical surgery on a lower second molar. After discussion with the patient, we decided to perform a reimplantation.
The tooth was periodontally sound, and exhibited normal mobility. It was not tender to percussion or palpation. Radiographic examination revealed a lucency associated with both roots, lucency in the furcation region and extruded material associated with both roots. Prior to extraction, the tooth is taken out of occlusion. When the tooth is repositioned, swelling may result in the tooth slightly extruding from the socket, so it is important to ensure there is good clearance from opposing teeth. Pre- & post-operative broad spectrum antibiotics such as amoxycillin are given. Chlorhexidine mouthrinse three times daily is initiated one day prior to the procedure and continued for one week after.
LA is given and the tooth carefully extracted. By this I mean that very gentle force is utilised. The tooth is grasped with forceps only by the crown. The root surface is not touched, so as to avoid damaging the cementum layer. It is common to spend 15 or 20 minutes extracting a tooth that might otherwise be extracted very rapidly. Obviously, the use of luxators and elevators is avoided.
Once extracted, I place a rubber band around the handles of the forceps to prevent the tooth being dropped. It’s important to keep the PDL moist, so have plenty of solution available and keep your assistant active in syringing solution over the PDL. A physiological solution is used. There is evidence that solutions such as hartman’s solution provide better stability of the PDL cells, but saline can also be used (a solution used in the transport of organs for transplant has been shown to by the best at maintaining viability of PDL cells, but this may be impractical).
The apicectomy is then performed using a high speed bur with copious water coolant. I know that water is not the best thing to be applying to PDL, but I think it’s more important to have coolant that to allow uncontrolled heat to be applied to the root. In general aim to remove 3mm of root tip. This should remove most of the accessory canals that may be harbouring microorganisms.
The next step is to use a small round bur to complete the retropreparation. There is no trick to this. Simply create a preparation in the root canal space 3-4mm deep. Any isthmus which is present between canals should also be prepared. This will especially occur in the mesial root of the lower molar. This preparation is designed to remove any infected GP and dentine and allow a retrofill to be placed which will seal in any remaining microorganisms or their products. Inspect the root surface for lateral canals and fill these in the same manner. In this case, the distal canal had been filled with MTA. MTA seals extremely well (compared to GP, which does allow micororganisms to pass) so there was no need to place a retrofill in the distal root.
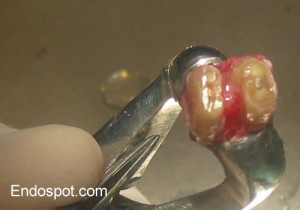
The retropreparation has been completed in the mesial root. The distal root is filled with MTA so no retrofill is required.
There are many suitable materials for retro filling. The two most popular with endodontists are MTA and Super EBA. These materials have shown a good ability to seal and MTA in particular is highly biocompatible. MTA can be diifcult to handle, so make sure you have practised mixing and handling it first if you plan to use this. If these materials are not available, then IRM may be used. In the past amalgam has been used for retrofilling, but this material tends to corode over time, leading to loss of seal. When placing the retro fill, pack the material into the cavity with a small amalgam plugger, and then burnish.
The next step is to carefully debride the pathological tissue from the tooth socket. Here, it is important to try to avoid disturbing the PDL. By debriding, you are also removing the blood clot which may have formed in the socket. Irrigate the socket thoroughly and reposition the tooth with gentle pressure. It helps to have the patient bite gently on a wooden spatula to help ensure the tooth is correctly positioned into the socket.
Generally, no splinting will be required and a suture is placed over the tooth to hold it in position. Splinting is not desired as it impedes cleaning the area, and may lead to higher rates of resorption. If the tooth is excessively mobile after replantation, then consider splinting for a short period (3-4 days). Again, I’m aware that guidelines for traumatically avulsed teeth advise splinting, but a replanted lower molar has much greater primary stability than a replanted central or lateral incisor which was knocked from the mouth, and years of experience by endodontists conducting this procedure show that splinting is rarely required.
A radiograph is taken immediately after replantation to help confirm that the tooth is repositioned and as a baseline for healing. The sutures can be removed after 3 days and occlusion checked again. At this point some physiological movement due to occlusion is fine, but make sure the tooth is not high in occlusion. Most patients report very little discomfort after the procedure. Oral hygiene is important and flossing and brushing should resume as soon as the patient is comfortable.
The post-operative radiograph revealed I had failed to remove the extruded sealer which was associated with the mesial root. This was probably a result of careful debridement of the socket. The decision was made to leave this in place as re-extracting the tooth was likely to case more damage than benefit.
Review of the case mentioned above at 2 weeks revealed the tooth to be quite mobile. This is not unusual, as is not a cause for concern as the tooth will generally become less mobile. Importantly, periodontal examination was within normal limits. indicating that reattachment of the PDL had been successful. The sinus tract had healed. Examination at three months revealed no sinus tract, nil tenderness to percussion nor palpation, normal periodontal examination and normal mobility. Radiographic examination revealed excellent healing of the periapical areas. There continued to be lucency in the furcation area. This tooth will be monitored and I’ll update the post accordingly.
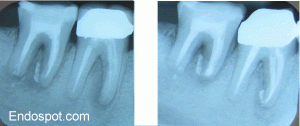
Pre-op and 3 Month Review Radiographs. Excellent, But Incomplete Healing of the PA Lesions are Apparent. Prognosis is Excellent. The First Molar Has Been Re-treated and Initial Healing of The PA Lesions is Also Apparent.

Six month review shows osseous healing adjacent to the root ends of the second molar. The tooth is functional, periodontally sound and mobility is normal. Coronal restoration should now proceed. The lesion on the mesial root of the first molar has also continued to heal but a lucency remains around the extruded sealer.
A three year review has now been undertaken. The tooth is asymptomatic, periodontal probing and mobility is normal. At this stage there appears to be no sign of ankylosis. The PA shows excellent resolution of the apical lucencies.
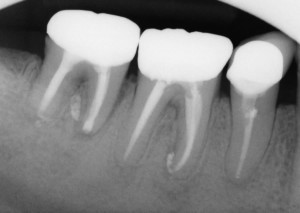
The three year review PA shows good healing of the apical lucency. The lucency associated with the mesial root of the first molar has also resolved. This simple and inexpensive treatment has allowed this patient to keep their tooth in function in the short term. I will keep recalling the patient.
I’d like to hear from others on your technique and experiences with IR. Feel free to send through cases and I’ll add them to this post.
Bender IB, Rossman LE (1993). Intentional replantation of endodontically treated teeth. Oral Surgery, Oral Medicine, Oral Pathology 76(5):623-630
Peer M (2004) Intentional replantation – A last resort treatment or a conventional treatment procedure? Nine case reports. Dental Traumatology 20:48-55
The best known reference for IR is Bender IB, Rossman LE (1993). Intentional replantation of endodontically treated teeth. Oral Surgery, Oral Medicine, Oral Pathology 76(5):623-630. In a retrospective case series they followed 31 cases for 1 day to 22 years and reported an overall success rate of 80%.
Another good overview is provided by Peer, however this is a simple case series. Criteria for success/failure is not listed and no rates of success are given: Peer M (2004) Intentional replantation – A last resort treatment or a conventional treatment procedure? Nine case reports. Dental Traumatology 20:48-55
Primary Indications for IR are listed as:
Secondary Indications:
Contraindications for IR are:
Advantages:
Disadvantages: